7686-77-3
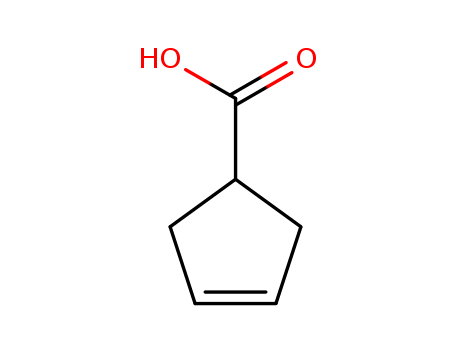
- Product Name:3-Cyclopentene-1-carboxylic acid
- Molecular Formula:C6H8O2
- Purity:98% GC
- Molecular Weight:112.128
Product Details
Manufacturer Supply Best Quality 3-Cyclopentene-1-carboxylic acid 7686-77-3 with Efficient Transportation
- Molecular Formula:C6H8O2
- Molecular Weight:112.128
- Appearance/Colour:Clear colorless to slightly yellow liquid
- Vapor Pressure:0.0282mmHg at 25°C
- Refractive Index:n20/D 1.469(lit.)
- Boiling Point:227.3 °C at 760 mmHg
- PKA:4.62±0.20(Predicted)
- Flash Point:101.1 °C
- PSA:37.30000
- Density:1.189 g/cm3
- LogP:1.03720
3-Cyclopentene-1-carboxylic acid(Cas 7686-77-3) Usage
InChI:InChI=1/C6H8O2/c7-6(8)5-3-1-2-4-5/h1-2,5H,3-4H2,(H,7,8)/p-1
7686-77-3 Relevant articles
N-SUBSTITUTED-DIOXOCYCLOBUTENYLAMINO-3-HYDROXY-PICOLINAMIDES USEFUL AS CCR6 INHIBITORS
-
Paragraph 0664; 0665, (2020/04/10)
The present invention relates to N-subst...
A green, economical synthesis of β-ketonitriles and trifunctionalized building blocks from esters and lactones
Pienaar, Daniel P.,Butsi, Kamogelo R.,Rousseau, Amanda L.,Brady, Dean
supporting information, p. 2930 - 2935 (2019/12/23)
The acylation of the acetonitrile anion ...
A Selective and Functional Group-Tolerant Ruthenium-Catalyzed Olefin Metathesis/Transfer Hydrogenation Tandem Sequence Using Formic Acid as Hydrogen Source
Zieliński, Grzegorz K.,Majtczak, Jaros?awa,Gutowski, Maciej,Grela, Karol
, p. 2542 - 2553 (2018/03/09)
A ruthenium-catalyzed transfer hydrogena...
Enantioselective desymmetrization via carbonyl-directed catalytic asymmetric hydroboration and Suzuki-Miyaura cross-coupling
Hoang, Gia L.,Yang, Zhao-Di,Smith, Sean M.,Miska, Judy L.,Prez, Damaris E.,Zeng, Xiao Cheng,Takacs, James M.,Pal, Rhitankar,Pelter, Libbie S. W.
supporting information, p. 940 - 943 (2015/03/30)
The rhodium-catalyzed enantioselective d...
7686-77-3 Process route
-
-
58101-60-3
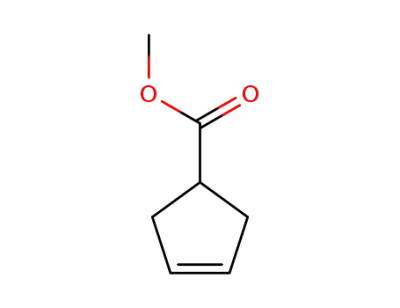
methyl cyclopent-3-ene-1-carboxylate

-
-
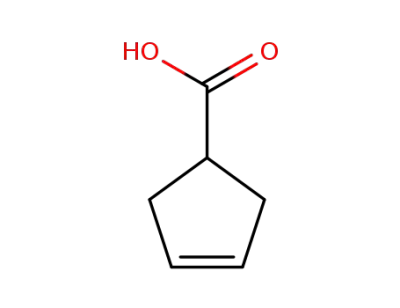
7686-77-3
3-cyclopentene-1-carboxylic acid
| Conditions | Yield |
|---|---|
|
With
water; lithium hydroxide;
In
tetrahydrofuran;
at 25 ℃;
for 15h;
|
90% |
-
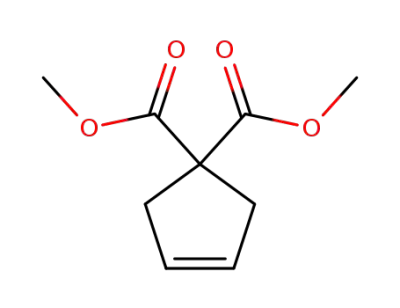
-
84646-68-4
Dimethyl 3-cyclopentene-1,1-dicarboxylate

-

-
7686-77-3
3-cyclopentene-1-carboxylic acid
| Conditions | Yield |
|---|---|
|
Dimethyl 3-cyclopentene-1,1-dicarboxylate;
With
water; potassium hydroxide;
In
ethanol;
at 45 ℃;
for 14h;
Inert atmosphere;
at 180 ℃;
for 1h;
Inert atmosphere;
|
73% |
|
Multi-step reaction with 2 steps
1: 45percent KOH / aq. ethanol / 15 h / Heating
2: 100 percent / 1 h / 185 - 195 °C
With
potassium hydroxide;
In
ethanol;
|
|
|
Multi-step reaction with 2 steps
1: water; potassium hydroxide / ethanol / 1 h / Reflux
2: 1 h / 180 °C / Neat (no solvent)
With
water; potassium hydroxide;
In
ethanol;
|
7686-77-3 Upstream products
-
88326-51-6
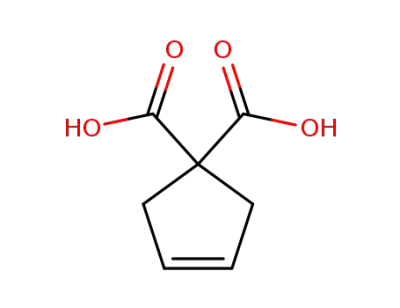
Dimethyl 3-cyclopentene-1,1-dicarboxylate
-
124-38-9
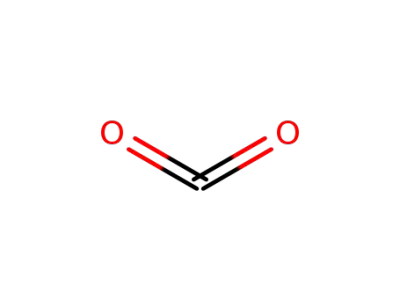
carbon dioxide
-
1781-66-4
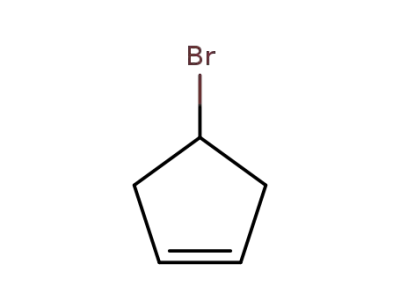
cyclopent-3-enyl bromide
-
7686-78-4
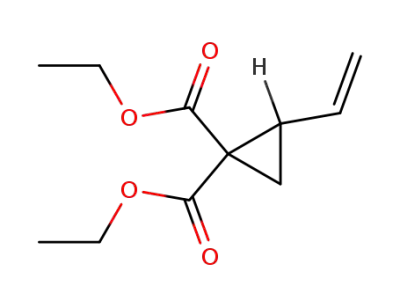
diethyl 2-vinylcyclopropane-1,1-dicarboxylate
7686-77-3 Downstream products
-
99-14-9
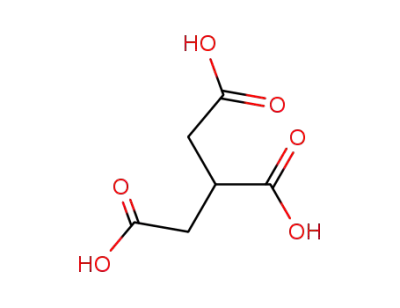
tricarallylic acid
-
25125-21-7
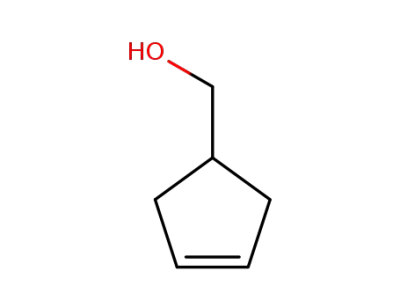
4-(hydroxymethyl)cyclopentene
-
89415-65-6
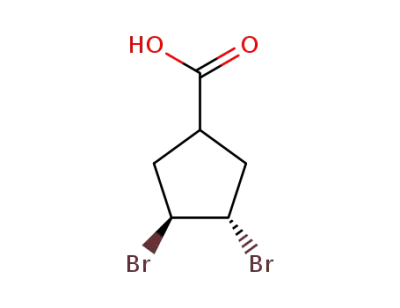
trans-3,4-dibromocyclopentane-1-carboxylic acid
-
5732-97-8
(1S,4R)-2-Oxa-bicyclo[2.2.1]heptan-3-one
Relevant Products
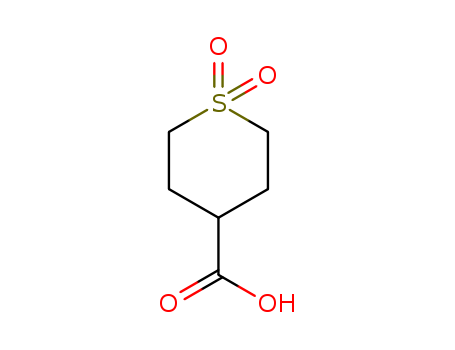
-
1,1-Dioxo-tetrahydrothiopyran-4-carboxylic acid
CAS:64096-87-3
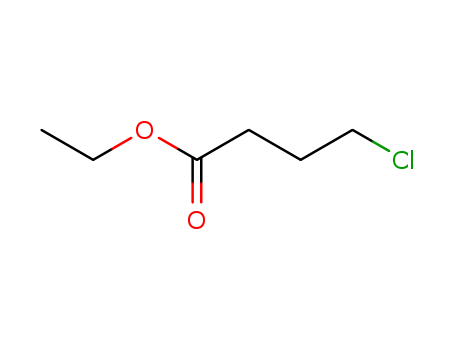
-
Ethyl 4-chlorobutyrate
CAS:3153-36-4
-
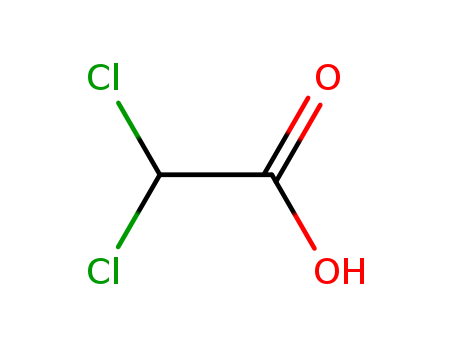
Dichloroacetic acid
CAS:79-43-6