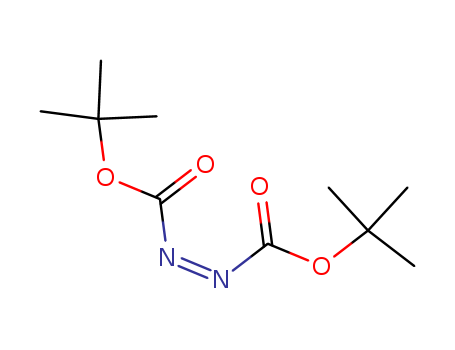
870-50-8
- Product Name:Di-tert-Butyl azodicarboxylate
- Molecular Formula:C10H18N2O4
- Purity:98% HPLC
- Molecular Weight:230.264
Product Details
Top Quality Chinese Factory supply 870-50-8 Di-tert-Butyl azodicarboxylate
- Molecular Formula:C10H18N2O4
- Molecular Weight:230.264
- Appearance/Colour:yellow crystals or crystalline powder
- Vapor Pressure:0.00253mmHg at 25°C
- Melting Point:89-92 °C
- Refractive Index:1.459
- Boiling Point:287.1 °C at 760 mmHg
- Flash Point:107.2 °C
- PSA:77.32000
- Density:1.06 g/cm3
- LogP:3.30880
Di-tert-Butyl azodicarboxylate(Cas 870-50-8) Usage
|
Purification Methods |
The tert-butyl ester has the advantage over the ethyl ester (below) in being a solid and more acid labile. It crystallises from ligroin and is best purified by covering the dry solid (22g) with pet ether (b 30-60o, 35-40 mL) heating to boiling and adding ligroin (b 60-90o) until the solid dissolves. On cooling, large lemon yellow crystals of the ester separate (~ 20g), m 90.7-92o. Evaporation of the filtrate gives a further crop of crystals [Carpino & Crowley Org Synth 44 18 1964]. This reagent is useful in the Mitsunobu reaction [Mitsunobu Synthesis 1 1981, Gennari et al. J Am Chem Soc 108 6394 1986, Evans et al. J Am Chem Soc 108 6394 1986, Hughes Org React 42 335 1992, Dodge et al. Org Synth 73 110 1996, Hughes Org Prep Proc Int 28 127 1996, Ferguson & Marcelle J Am Chem Soc 128 4576 2006, see also DEAD and DIAD below]. |
|
General Description |
Di-tert-Butyl azodicarboxylate (DBAD) is a key reagent used in Mitsunobu reactions, where it acts as an oxidizing agent alongside triphenylphosphine to facilitate the conversion of alcohols into esters or other functional groups. It is also employed in copper-catalyzed tandem oxidation-olefination processes, demonstrating its versatility in organic synthesis for constructing complex molecules under mild, environmentally friendly conditions. Its role in these reactions highlights its importance in facilitating selective transformations, such as the synthesis of sulfoxides, sulfones, and alkenes, while maintaining compatibility with various functional groups. |
InChI:InChI=1/C10H18N2O4/c1-9(2,3)15-7(13)11-12-8(14)16-10(4,5)6/h1-6H3/b12-11+
870-50-8 Relevant articles
Bifunctional Molecular Probes for Activity-Based Visualization of Quinone-Dependent Amine Oxidases
Burke, Ashley A.,Barrows, Luke,Solares, Maria J.,Wall, Alexander D.,Jakobsche, Charles E.
, p. 17681 - 17685 (2018)
The design, synthesis, and evaluation of...
Hydrogen peroxide based oxidation of hydrazines using HBr catalyst
Du, Wanting,Ma, Zichao,Shao, Liming,Wang, Jian
, (2021/11/18)
Azo compounds (RN = NR′) are an importan...
Synthesis of 1,2-Dihydroquinolines via Hydrazine-Catalyzed Ring-Closing Carbonyl-Olefin Metathesis
Zhang, Yunfei,Sim, Jae Hun,Macmillan, Samantha N.,Lambert, Tristan H.
supporting information, p. 6026 - 6030 (2020/08/05)
The synthesis of 1,2-dihydroquinolines b...
Synthesis of α-aminocarbonyl compounds via hetero dielsalder reaction
Sakurai, Masayoshi,Kihara, Nobuhiro,Watanabe, Nobuhiro,Ikari, Yoshihiro,Takata, Toshikazu
supporting information, p. 144 - 147 (2018/01/01)
A synthetic route to α-aminoketone deriv...
870-50-8 Process route
-
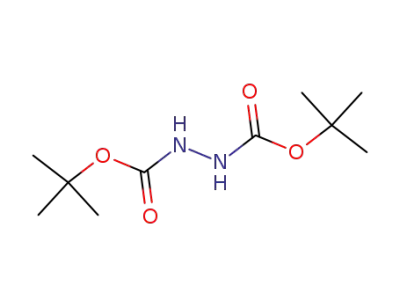
-
16466-61-8
1,2-bis(t-butyloxycarbonyl)hydrazine

-
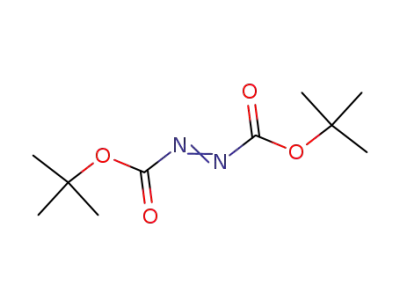
-
870-50-8
di-tert-butyl-diazodicarboxylate
| Conditions | Yield |
|---|---|
|
With
pyridine; bromine;
In
dichloromethane;
at 0 ℃;
for 0.25h;
|
100% |
|
With
hydrogen bromide; dihydrogen peroxide;
In
1,4-dioxane; water;
at 50 ℃;
for 0.5h;
|
94% |
|
With
pyridine; bromine;
In
dichloromethane;
at 0 ℃;
for 1.5h;
Inert atmosphere;
|
92% |
|
With
pyridine; bromine;
In
dichloromethane;
at 0 ℃;
for 0.75h;
|
87% |
|
With
pyridine; bromine;
In
dichloromethane;
for 0.666667h;
Cooling with ice;
|
86% |
|
With
pyridine; bromine;
In
dichloromethane;
Inert atmosphere;
|
81% |
|
With
pyridine; bromine;
In
dichloromethane;
|
81% |
|
With
pyridine; bromine;
In
dichloromethane;
at 0 ℃;
for 0.5h;
|
80% |
|
With
pyridine; N-Bromosuccinimide;
In
dichloromethane;
|
|
|
With
oxygen; copper;
at 20 ℃;
for 3h;
|
|
|
With
pyridine; tert-butylhypochlorite;
In
dichloromethane;
at 0 ℃;
for 1h;
|
-
-
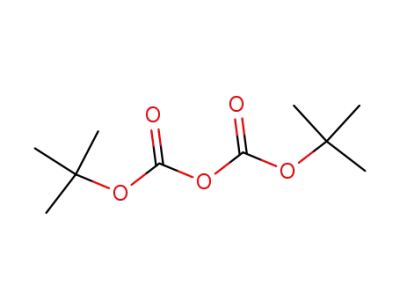
24424-99-5
di-tert-butyl dicarbonate

-

-
870-50-8
di-tert-butyl-diazodicarboxylate
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 2 steps
1: hydrazine hydrate / methanol / Inert atmosphere
2: pyridine; bromine / dichloromethane / Inert atmosphere
With
pyridine; bromine; hydrazine hydrate;
In
methanol; dichloromethane;
|
|
|
Multi-step reaction with 2 steps
1: hydrazine hydrate / methanol
2: pyridine; bromine / dichloromethane
With
pyridine; bromine; hydrazine hydrate;
In
methanol; dichloromethane;
|
|
|
Multi-step reaction with 2 steps
1: hydrazine hydrate / methanol / -10 - 20 °C
2: bromine; pyridine / dichloromethane / 0.5 h / 0 °C
With
pyridine; bromine; hydrazine hydrate;
In
methanol; dichloromethane;
|
870-50-8 Upstream products
-
16466-61-8

1,2-bis(t-butyloxycarbonyl)hydrazine
-
28899-97-0
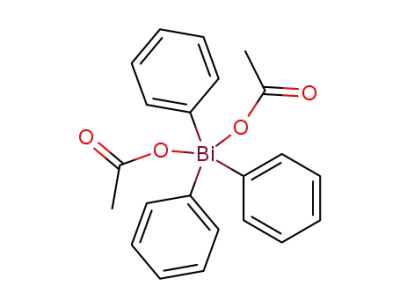
triphenylbismuth(V) diacetate
-
24424-99-5

di-tert-butyl dicarbonate
-
24608-52-4
tert-butyl chloroformate
870-50-8 Downstream products
-
22390-25-6
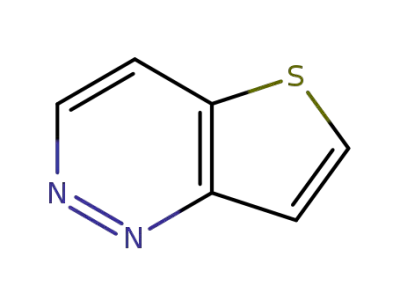
Thieno<3,2-c>pyridazin
-
60226-19-9
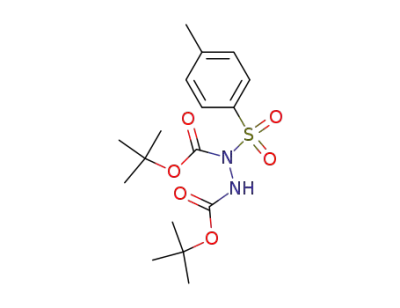
di-tert-butyl 1-tosylhydrazine-1,2-dicarboxylate
-
144823-64-3
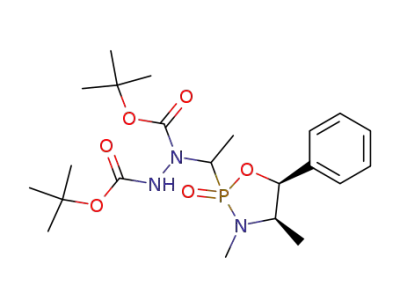
C22H36N3O6P
-
115171-06-7
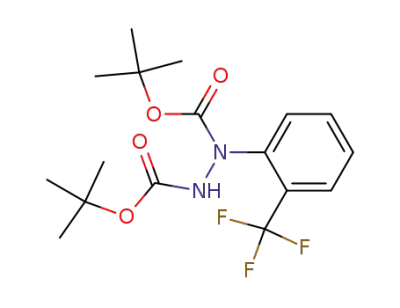
C17H23F3N2O4
Relevant Products
-
2-Bromoacetamide
CAS:683-57-8
-
6-Methylheptanoic acid
CAS:929-10-2
-
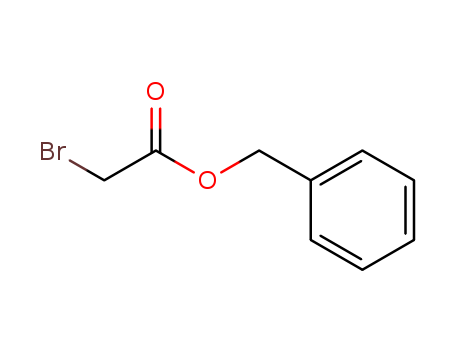
Benzyl 2-bromoacetate
CAS:5437-45-6