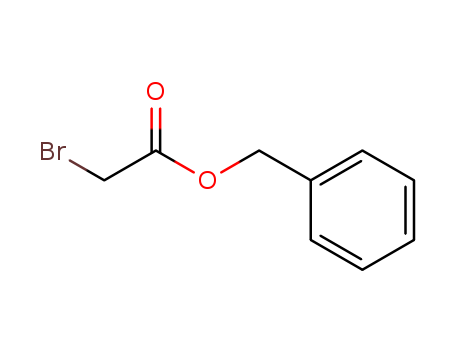
5437-45-6
- Product Name:Benzyl 2-bromoacetate
- Molecular Formula:C9H9BrO2
- Purity:98% GC
- Molecular Weight:229.073
Product Details
Reliable factory customized supply Benzyl 2-bromoacetate 5437-45-6
- Molecular Formula:C9H9BrO2
- Molecular Weight:229.073
- Appearance/Colour:Colorless or light yellow liquid
- Vapor Pressure:0.00243mmHg at 25°C
- Melting Point:199-201 °C(Solv: water (7732-18-5))
- Refractive Index:n20/D 1.544(lit.)
- Boiling Point:287.773 °C at 760 mmHg
- Flash Point:116.485 °C
- PSA:26.30000
- Density:1.474 g/cm3
- LogP:2.12470
Benzyl 2-bromoacetate(Cas 5437-45-6) Usage
|
Purification Methods |
Dilute the ester with Et2O, wash it with 10% aqueous NaHCO3, H2O, dry (MgSO4) and fractionate it using a Fenske (glass helices packing) column. [Bergmann & Szinai J Chem Soc 1521 1956, Beilstein 6 IV 2265.] LACHRYMATORY. |
InChI:InChI=1/C9H9BrO2/c10-6-9(11)12-7-8-4-2-1-3-5-8/h1-5H,6-7H2
5437-45-6 Relevant articles
Fmoc-based synthesis of glycolate ester peptides for the assembly of de novo designed multimeric proteins using subtiligase
Suich, Daniel J.,Ballinger, Marcus D.,Wells, James A.,DeGrado, William F.
, p. 6653 - 6656 (1996)
An automated method utilizing Fmoc-prote...
Engineering Dirhodium Artificial Metalloenzymes for Diazo Coupling Cascade Reactions**
Bultman, Max J.,Huang, Rui,Lewis, Jared C.,Li, Ying,Roux, Benoit,Upp, David M.
supporting information, p. 23672 - 23677 (2021/08/23)
Artificial metalloenzymes (ArMs) are com...
Visible-Light-Induced Oxidative α-Alkylation of Glycine Derivatives with Ethers under Metal-Free Conditions
Song, Yang,Zhang, Hao,Guo, Jiabao,Shao, Yifei,Ding, Yuzhou,Zhu, Li,Yao, Xiaoquan
, p. 5914 - 5921 (2021/11/22)
In this work, a visible-light-induced ox...
1,5-Disubstituted 1,2,3-Triazoles as Amide Bond Isosteres Yield Novel Tumor-Targeting Minigastrin Analogs
Grob, Nathalie M.,Schibli, Roger,Béhé, Martin,Valverde, Ibai E.,Mindt, Thomas L.
supporting information, p. 585 - 592 (2021/04/12)
1,5-Disubstituted 1,2,3-triazoles (1,5-T...
E- and chemoselective thia-Michael addition to benzyl allenoate
Bibi, Rifhat,Murtaza, Amna,Khan, Khalid Mohammed,Rehman, Zia ur,Saeed, Aamer,Tahir, Muhammad Nawaz,Hassan, Abbas
supporting information, p. 969 - 975 (2020/08/05)
Different thiols were successfully react...
5437-45-6 Process route
-

-
103-50-4
dibenzyl ether

-
-
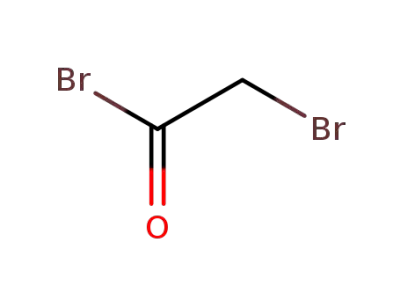
598-21-0
2-Bromoacetyl bromide

-
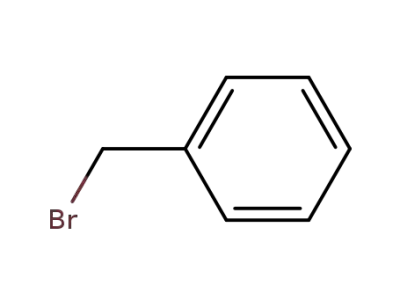
-
100-39-0
benzyl bromide

-
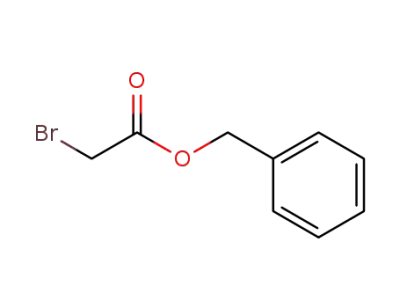
-
5437-45-6
Benzyl bromoacetate
| Conditions | Yield |
|---|---|
|
at 100 ℃;
for 10h;
Product distribution;
|
-

-
598-21-0
2-Bromoacetyl bromide

-
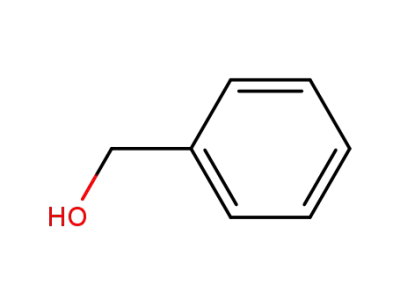
-
100-51-6,185532-71-2
benzyl alcohol

-

-
5437-45-6
Benzyl bromoacetate
| Conditions | Yield |
|---|---|
|
With
sodium hydrogencarbonate;
In
acetonitrile;
at 0 ℃;
for 0.916667h;
Sealed tube;
Inert atmosphere;
|
98% |
|
With
dmap;
In
dichloromethane;
at 20 ℃;
for 2h;
Inert atmosphere;
|
94% |
|
With
2,6-dimethylpyridine;
In
dichloromethane;
at 0 ℃;
for 2h;
|
91% |
|
With
sodium hydrogencarbonate;
In
water; acetonitrile;
at 0 - 20 ℃;
for 0.666667h;
|
81% |
|
With
sodium hydrogencarbonate;
In
acetonitrile;
at 0 - 25 ℃;
for 5h;
|
71% |
|
With
sodium hydrogencarbonate;
In
acetonitrile;
at 23 ℃;
for 1h;
Inert atmosphere;
|
60% |
|
With
sodium hydrogencarbonate;
In
acetonitrile;
at 20 ℃;
|
|
|
In
dichloromethane;
at 0 - 20 ℃;
|
|
|
With
triethylamine;
In
dichloromethane;
at 0 - 20 ℃;
|
|
|
benzyl alcohol;
With
sodium hydrogencarbonate;
In
dichloromethane;
at 0 ℃;
for 0.0833333h;
Schlenk technique;
Inert atmosphere;
2-Bromoacetyl bromide;
In
dichloromethane;
for 3.5h;
Schlenk technique;
Inert atmosphere;
|
|
|
With
pyridine;
In
dichloromethane;
at 0 - 25 ℃;
for 0.833333h;
|
|
|
With
dmap; triethylamine;
In
dichloromethane;
at 0 - 20 ℃;
for 3h;
|
|
|
With
sodium hydrogencarbonate;
In
acetonitrile;
at 0 - 4 ℃;
for 0.75h;
|
|
|
With
sodium hydrogencarbonate;
In
acetonitrile;
at 0 - 4 ℃;
for 0.75h;
|
|
|
With
pyridine;
In
dichloromethane;
at 0 - 25 ℃;
for 0.833333h;
|
|
|
With
sodium hydrogencarbonate;
In
acetonitrile;
at 0 ℃;
for 0.166667h;
|
|
|
With
sodium hydrogencarbonate;
In
acetonitrile;
at 0 ℃;
for 0.5h;
|
|
|
With
sodium hydrogencarbonate;
In
acetonitrile;
at 0 ℃;
for 0.5h;
|
|
|
With
sodium hydrogencarbonate;
In
acetonitrile;
at 0 - 4 ℃;
for 0.75h;
|
|
|
With
sodium hydrogencarbonate;
In
acetonitrile;
at 0 ℃;
for 0.5h;
|
|
|
With
pyridine;
In
dichloromethane;
at 0 - 20 ℃;
|
|
|
With
sodium hydrogencarbonate;
In
acetonitrile;
at 0 ℃;
for 0.166667h;
Inert atmosphere;
|
|
|
With
sodium hydrogencarbonate;
In
acetonitrile;
at 0 ℃;
for 0.166667h;
Inert atmosphere;
|
|
|
With
pyridine;
In
dichloromethane;
at 0 - 20 ℃;
|
|
|
With
sodium hydrogencarbonate;
In
acetonitrile;
at 0 - 20 ℃;
for 0.25h;
Inert atmosphere;
|
|
|
With
triethylamine;
In
dichloromethane;
at 0 - 20 ℃;
|
5437-45-6 Upstream products
-
79-08-3
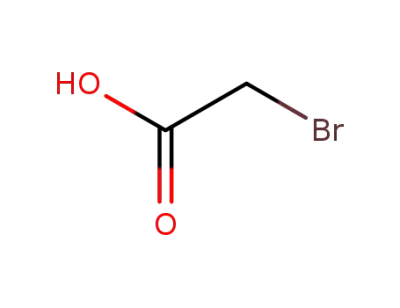
bromoacetic acid
-
100-51-6

benzyl alcohol
-
105-36-2
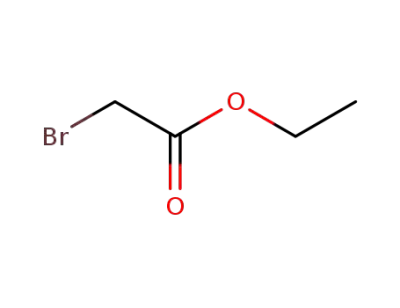
ethyl bromoacetate
-
13837-45-1
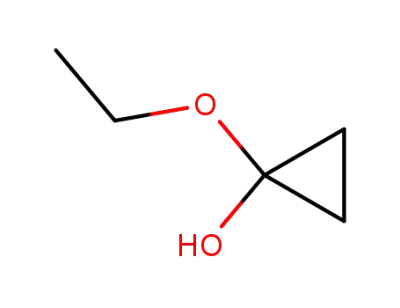
1-ethoxy-1-cyclopropanol
5437-45-6 Downstream products
-
13474-22-1
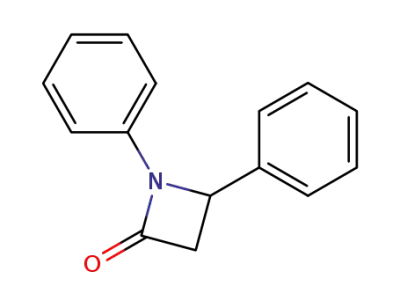
1,4-diphenyl-azetidin-2-one
-
88390-01-6
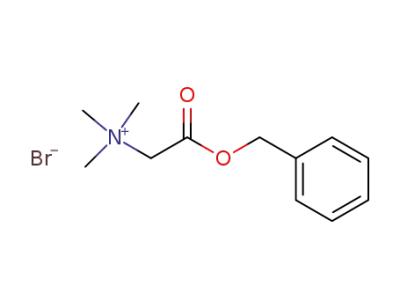
Trimethylammoniumessigsaeurebetain-benzylester-bromid
-
409109-80-4
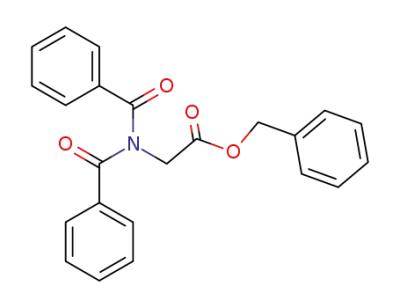
N,N-dibenzoyl-glycine benzyl ester
-
58365-10-9
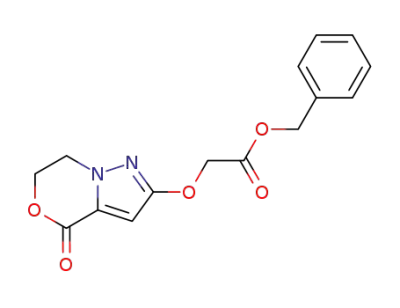
(4-oxo-6,7-dihydro-4H-pyrazolo[5,1-c][1,4]oxazin-2-yloxy)-acetic acid benzyl ester
Relevant Products
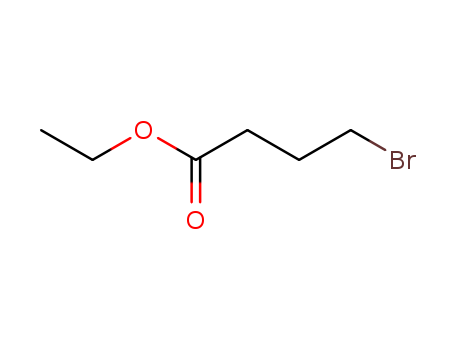
-
Ethyl 4-bromobutyrate
CAS:2969-81-5
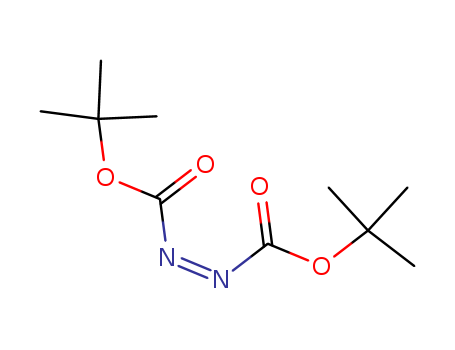
-
Di-tert-Butyl azodicarboxylate
CAS:870-50-8
-
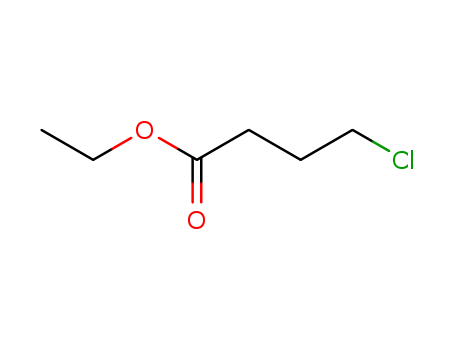
Ethyl 4-chlorobutyrate
CAS:3153-36-4