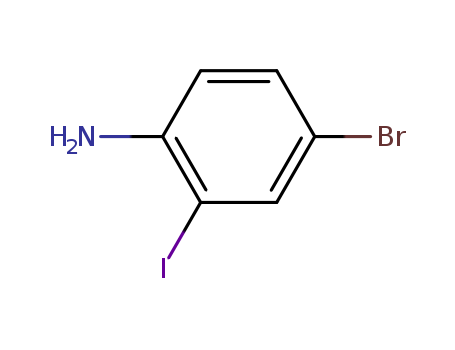
66416-72-6
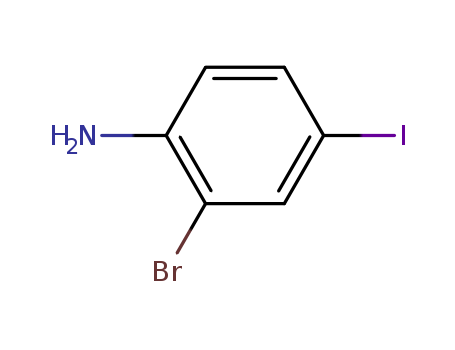
- Product Name:4-Bromo-2-iodoaniline
- Molecular Formula:C6H5 Br I N
- Purity:98% GC
- Molecular Weight:297.921
Product Details
Factory Export Top Purity 4-Bromo-2-iodoaniline 66416-72-6 In Stock
- Molecular Formula:C6H5 Br I N
- Molecular Weight:297.921
- Appearance/Colour:Grey to purple powder
- Vapor Pressure:0.00131mmHg at 25°C
- Melting Point:69-72oC(lit.)
- Boiling Point:297.9°C at 760 mmHg
- PKA:1.83±0.10(Predicted)
- Flash Point:134°C
- PSA:26.02000
- Density:2.292g/cm3
- LogP:3.21710
4-BROMO-2-IODOANILINE(Cas 66416-72-6) Usage
|
General Description |
4-Bromo-2-iodoaniline is a 2-iodoaniline derivative. It can be prepared by reacting 4-bromoaniline with iodine. |
InChI:InChI=1/C6H5BrIN/c7-4-1-2-6(9)5(8)3-4/h1-3H,9H2
66416-72-6 Relevant articles
CYCLOPROPYL DIHYDROQUINOLINE SULFONAMIDE COMPOUNDS
-
Paragraph 0233-0234; 0280-0281, (2021/12/29)
The present invention provides a compoun...
Modular counter-Fischer?indole synthesis through radical-enolate coupling
Chung, Hyunho,Kim, Jeongyun,Gonzalez-Montiel, Gisela A.,Cheong, Paul Ha-Yeon,Lee, Hong Geun
supporting information, p. 1096 - 1102 (2021/01/26)
A single-electron transfer mediated modu...
Cu(II)-Promoted Cascade Synthesis of Fused Imidazo-Pyridine-Carbonitriles
Rakshit, Amitava,Dhara, Hirendra Nath,Alam, Tipu,Dahiya, Anjali,Patel, Bhisma K.
supporting information, p. 17504 - 17510 (2021/11/18)
A Cu(II)-promoted synthesis of an aza-fu...
CYCLOBUTYL DIHYDROQUINOLINE SULFONAMIDE COMPOUNDS
-
, (2021/12/29)
The present invention provides a cyclobu...
66416-72-6 Process route
-
-
106-40-1
4-bromo-aniline

-
-
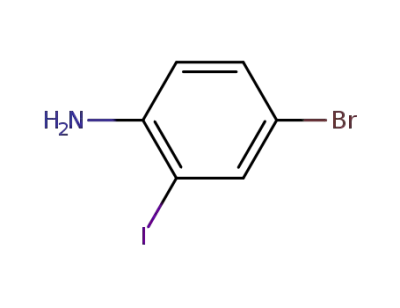
66416-72-6
4-bromo-2-iodoaniline

-
-
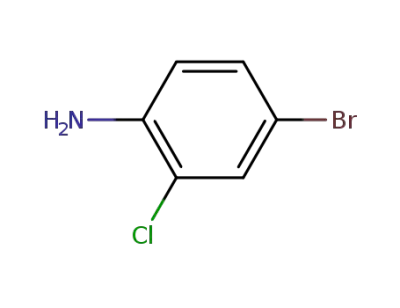
38762-41-3
2-chloro-4-bromoaniline
| Conditions | Yield |
|---|---|
|
With
1-butyl-3-methyl-pyridinium dichloroiodate;
for 2h;
Time;
|
99 %Chromat. 98 %Chromat. |
-

-
106-40-1
4-bromo-aniline

-

-
66416-72-6
4-bromo-2-iodoaniline
| Conditions | Yield |
|---|---|
|
With
N,N,N-trimethylbenzenemethanaminium dichloroiodate; calcium carbonate;
In
methanol; dichloromethane;
for 8h;
Ambient temperature;
|
89% |
|
With
1,4-dibenzyl-1,4-diazoniabicyclo[2.2.2]octane dichloroiodate;
In
neat (no solvent);
at 20 ℃;
for 0.416667h;
regioselective reaction;
|
89% |
|
With
4O4S(2-)*8Na(1+)*2H2O2*NaCl; acetic acid; potassium iodide;
at 50 - 52 ℃;
for 6h;
regioselective reaction;
Green chemistry;
|
89% |
|
With
N-iodo-succinimide; acetic acid;
at 23 ℃;
for 1h;
|
88% |
|
With
N,N,N-trimethylbenzenemethanaminium dichloroiodate; calcium carbonate;
In
methanol; dichloromethane;
|
81% |
|
With
hydrogenchloride; potassium iodate; potassium iodide;
In
methanol; water;
|
81% |
|
4-bromo-aniline;
With
iodine;
In
cyclohexane;
at 50 ℃;
for 0.5h;
With
dihydrogen peroxide;
In
cyclohexane;
at 50 ℃;
for 4h;
|
75% |
|
4-bromo-aniline;
With
iodine;
In
cyclohexane;
at 50 ℃;
for 0.5h;
With
dihydrogen peroxide;
In
cyclohexane; water;
at 50 ℃;
for 4h;
|
75% |
|
4-bromo-aniline;
With
iodine;
In
cyclohexane;
at 50 ℃;
for 0.5h;
With
dihydrogen peroxide;
In
cyclohexane; water;
at 50 ℃;
for 4h;
|
75% |
|
With
iodine;
In
tert-butyl methyl ether; dimethyl sulfoxide;
at 20 ℃;
for 2.5h;
Solvent;
regioselective reaction;
|
71% |
|
With
ammonium iodide; trans-3,5-dihydroperoxy-3,5-dimethyl-1,2-dioxolane;
In
acetonitrile;
at 20 ℃;
for 4h;
regioselective reaction;
|
65% |
|
4-bromo-aniline;
With
iodine;
In
cyclohexane;
at 50 ℃;
With
dihydrogen peroxide; sodium sulfite;
In
cyclohexane; water;
|
65% |
|
With
iodine; silver sulfate;
In
ethanol;
|
48% |
|
With
iodine; urea hydrogen peroxide adduct;
In
chloroform;
for 0.166667h;
microwave irradiation;
|
40% |
|
With
iodine; periodic acid;
In
dichloromethane;
for 0.166667h;
Heating;
microwave irradiation;
|
11% |
|
With
diethyl ether; water; iodine; calcium carbonate;
|
|
|
With
iodine; silver sulfate;
In
ethanol;
at 20 ℃;
for 16h;
|
|
|
With
Iodine monochloride;
In
N,N-dimethyl-formamide;
at 20 ℃;
for 0.5h;
|
|
|
With
benzyltrimethylammonium chloride; calcium carbonate;
In
methanol; chloroform;
at 20 ℃;
for 24h;
|
|
|
With
iodine; sodium hydrogencarbonate;
In
water;
cooling;
|
|
|
With
iodine; sodium hydrogencarbonate;
In
water;
at 20 ℃;
for 2.16667h;
|
|
|
With
iodine; sodium hydrogencarbonate;
In
water;
at 5 - 10 ℃;
for 12h;
|
|
|
With
iodine;
In
dimethyl sulfoxide;
at 100 ℃;
for 4h;
|
|
|
With
N-iodo-succinimide;
In
acetic acid;
at 20 ℃;
regioselective reaction;
|
|
|
With
iodine; sodium hydrogencarbonate;
In
water; toluene;
at 20 ℃;
Inert atmosphere;
|
|
|
4-bromo-aniline;
With
sodium hydrogencarbonate;
In
toluene;
at 20 ℃;
for 0.25h;
Schlenk technique;
Inert atmosphere;
With
iodine;
In
toluene;
at 20 ℃;
for 0.5h;
Schlenk technique;
Inert atmosphere;
|
|
|
With
iodine; sodium hydrogencarbonate;
In
water; toluene;
at 20 ℃;
for 6h;
|
|
|
With
dihydrogen peroxide; iodine;
|
66416-72-6 Upstream products
-
106-40-1

4-bromo-aniline
-
615-43-0
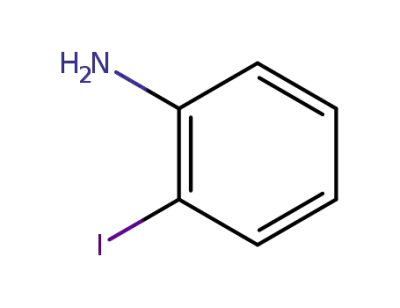
2-iodophenylamine
-
62-53-3
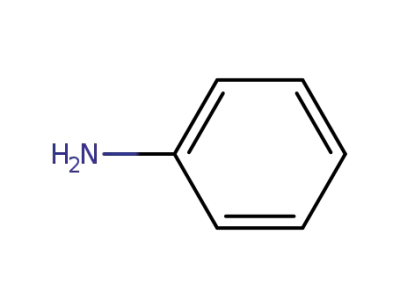
aniline
66416-72-6 Downstream products
-
89280-77-3
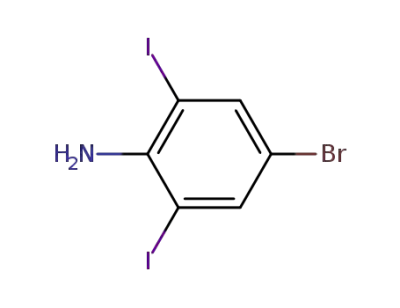
4-bromo-2,6-diiodoaniline
-
562080-91-5
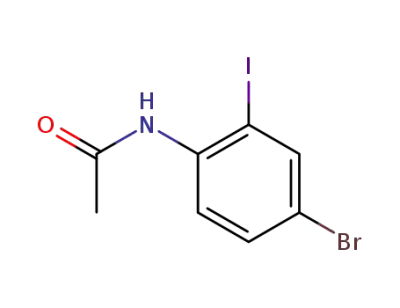
N-(4-bromo-2-iodophenyl)acetamide
-
112671-47-3
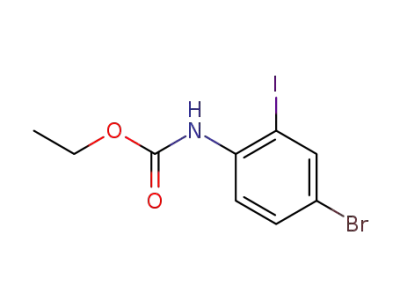
ethyl (4-bromo-2-iodophenyl)carbamate
-
5304-21-2
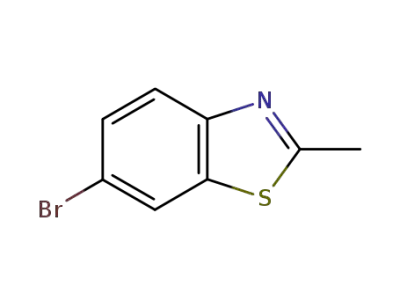
6-bromo-2-methylbenzothiazole
Relevant Products
-
2-Bromo-4-iodoaniline
CAS:29632-73-3
-
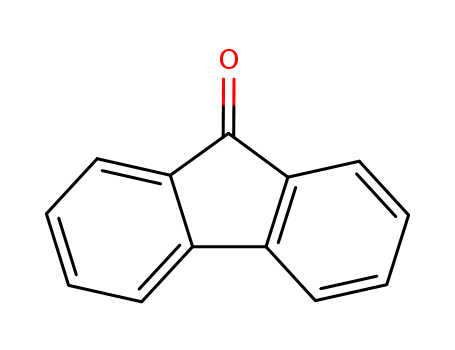
9-Fluorenone
CAS:486-25-9
-
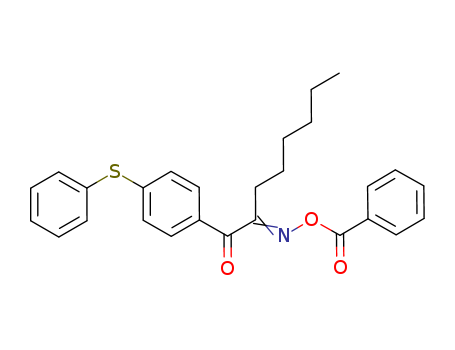
1-[4-(Phenylthio)phenyl]-1,2-octanedione 2-(O-benzoyloxime)
CAS:253585-83-0