58101-60-3
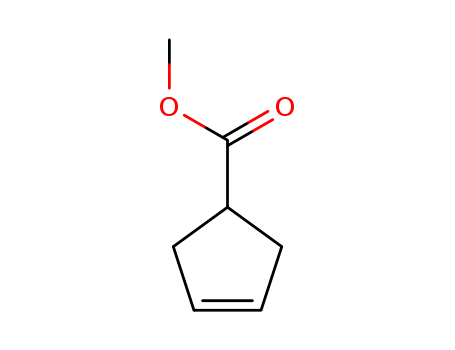
- Product Name:Methyl 3-cyclopentenecarboxylate
- Molecular Formula:C7H10O2
- Purity:98% GC
- Molecular Weight:126.155
Product Details
Factory Export Top Purity Methyl 3-cyclopentenecarboxylate 58101-60-3 In Stock
- Molecular Formula:C7H10O2
- Molecular Weight:126.155
- Vapor Pressure:3.95mmHg at 25°C
- Refractive Index:1.473
- Boiling Point:149.8 ºC at 760 mmHg
- Flash Point:33.8 ºC
- PSA:26.30000
- Density:1.054 g/cm3
- LogP:1.12560
Methyl 3-cyclopentenecarboxylate(Cas 58101-60-3) Usage
|
Use Description |
Methyl 3-cyclopentenecarboxylate is a chemical compound with versatile applications in various fields. In the realm of organic synthesis and chemical research, it serves as a valuable building block for the creation of complex organic molecules and pharmaceutical compounds, facilitating drug discovery and the development of potential therapeutic agents. Additionally, in the fragrance and flavor industry, Methyl 3-cyclopentenecarboxylate can be utilized to impart unique scents and flavors, enhancing the sensory experiences of perfumes, cosmetics, and food products. Its applications in organic synthesis and fragrance creation underscore its significance in driving innovation, scientific exploration, and product development within these distinct domains. |
InChI:InChI=1/C7H10O2/c1-9-7(8)6-4-2-3-5-6/h2-3,6H,4-5H2,1H3
58101-60-3 Relevant articles
-
Bond,F.T.,Ho,C.-Y.
, p. 1421 - 1425 (1976)
-
A FACILE SYNTHESIS OF (+/-)-SESBANINE VIA γ-ADDITION OF KETENE SILYL ACETAL WITH QUATERNIZED METHYL NICOTINATE
Wada, Makoto,Nishihara, Yoshihiro,Akiba, Kin-ya
, p. 3267 - 3270 (1985)
Total synthesis of (+/-)-sesbanine (1) w...
Synthesis and Conformational Aspects of cis- and trans-3-Carbomethoxy-6-oxabicyclohexane
Lizotte, Kathryn E.,Marecki, Paul E.,Mertes, Mathias P.
, p. 3594 - 3597 (1983)
-
Carbocyclic 3′-deoxyadenosine-based highly potent bisubstrate-analog inhibitor of basophilic protein kinases
Enkvist, Erki,Raidaru, Gerda,Vaasa, Angela,Pehk, T?nis,Lavogina, Darja,Uri, Asko
, p. 5336 - 5339 (2007)
Carbocyclic analogs of 3′-deoxyadenosine...
3-(1H-PYRAZOL-4-YL)PYRIDINE ALLOSTERIC MODULATORS OF THE M4 MUSCARINIC ACETYLCHOLINE RECEPTOR
-
Page/Page column 35, (2019/01/16)
The present invention is directed to pyr...
Copper-Catalyzed Modular Amino Oxygenation of Alkenes: Access to Diverse 1,2-Amino Oxygen-Containing Skeletons
Hemric, Brett N.,Chen, Andy W.,Wang, Qiu
supporting information, p. 1468 - 1488 (2019/01/25)
Copper-catalyzed alkene amino oxygenatio...
Hydroalkylation of Olefins to Form Quaternary Carbons
Green, Samantha A.,Huffman, Tucker R.,McCourt, Ruairí O.,Van Der Puyl, Vincent,Shenvi, Ryan A.
supporting information, (2019/05/22)
Metal-hydride hydrogen atom transfer (MH...
Hydroalkylation of Olefins to Form Quaternary Carbons
Green, Samantha A.,Huffman, Tucker R.,McCourt, Ruairí O.,Van Der Puyl, Vincent,Shenvi, Ryan A.
supporting information, p. 7709 - 7714 (2019/05/22)
Metal-hydride hydrogen atom transfer (MH...
58101-60-3 Process route
-
-
67-56-1
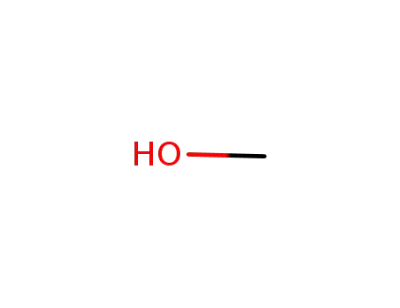
methanol

-
-
7686-77-3
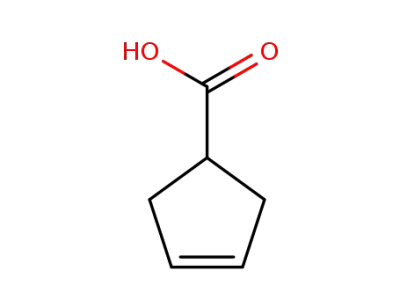
3-cyclopentene-1-carboxylic acid

-
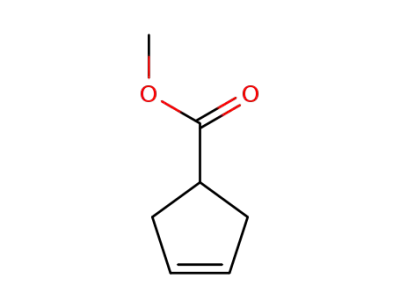
-
58101-60-3
methyl cyclopent-3-ene-1-carboxylate
| Conditions | Yield |
|---|---|
|
3-cyclopentene-1-carboxylic acid;
With
1,1'-carbonyldiimidazole;
In
dichloromethane;
at 0 - 20 ℃;
for 4h;
methanol;
In
dichloromethane;
|
99% |
|
With
oxalyl dichloride;
In
dichloromethane; N,N-dimethyl-formamide;
|
85% |
|
With
sulfuric acid;
at 0 - 80 ℃;
for 6h;
|
80% |
|
With
thionyl chloride;
at -70 - 0 ℃;
for 1h;
|
65% |
|
With
N-(3-dimethylaminopropyl)-N-ethylcarbodiimide;
|
53% |
|
With
sulfuric acid;
at 20 ℃;
for 16.5h;
|
-

-
7686-77-3
3-cyclopentene-1-carboxylic acid

-
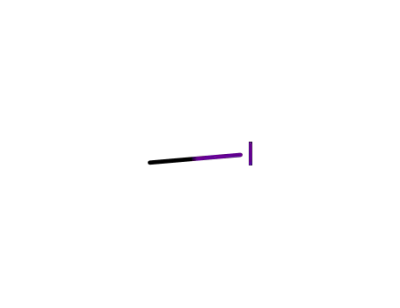
-
74-88-4
methyl iodide

-

-
58101-60-3
methyl cyclopent-3-ene-1-carboxylate
| Conditions | Yield |
|---|---|
|
With
potassium carbonate;
In
DMF (N,N-dimethyl-formamide);
at 20 ℃;
for 16h;
|
96% |
|
With
potassium carbonate;
In
N,N-dimethyl-formamide;
at 20 ℃;
for 16h;
|
96% |
|
With
potassium carbonate;
In
DMF (N,N-dimethyl-formamide);
at 20 ℃;
for 16h;
|
96% |
|
With
potassium carbonate;
In
N,N-dimethyl-formamide;
at 20 ℃;
for 16h;
Schlenk technique;
Inert atmosphere;
|
87% |
|
With
potassium carbonate;
In
N,N-dimethyl-formamide;
at 20 ℃;
for 16h;
|
86% |
58101-60-3 Upstream products
-
67-56-1

methanol
-
58191-35-8
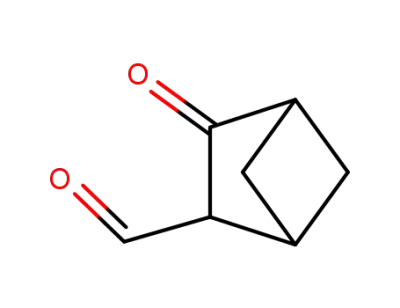
3-Formylbicyclo<2.1.1>hexan-2-on
-
3744-80-7
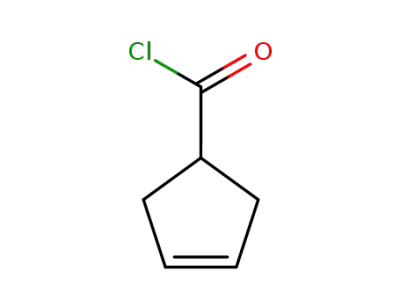
cyclopent-3-ene-1-carboxylic acid chloride
-
1587-26-4
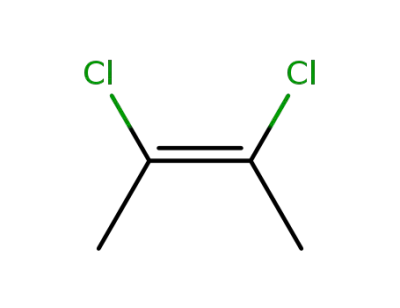
2,3-dichloro-2-butene, cis form
58101-60-3 Downstream products
-
67838-06-6
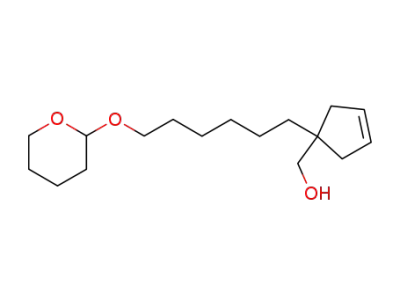
1-(6-tetrahydropyranyloxyhexyl)-1-hydroxymethylcyclopent-3-ene
-
75267-04-8
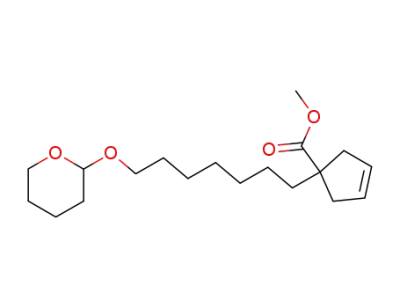
1-[7-(Tetrahydro-pyran-2-yloxy)-heptyl]-cyclopent-3-enecarboxylic acid methyl ester
-
131164-18-6
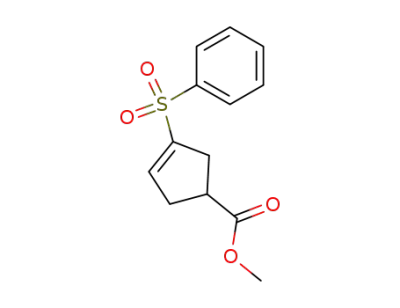
methyl 1-(phenylsulfonyl)cyclopentene-4-carboxylate
-
32811-76-0
3-hydroxycyclopentanecarboxylic acid methyl ester
Relevant Products
-
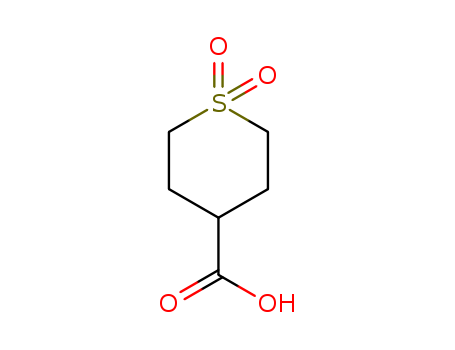
1,1-Dioxo-tetrahydrothiopyran-4-carboxylic acid
CAS:64096-87-3
-
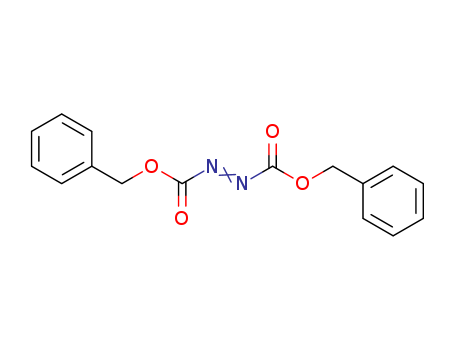
Dibenzyl azodicarboxylate
CAS:2449-05-0
-
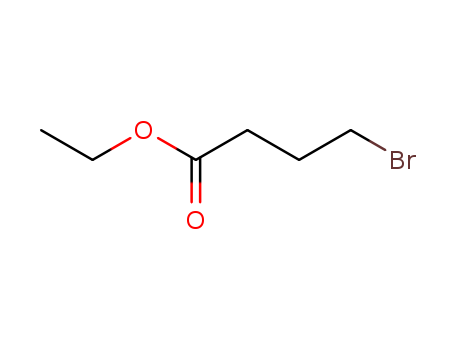
Methyl 2,3-dibromopropionate
CAS:1729-67-5