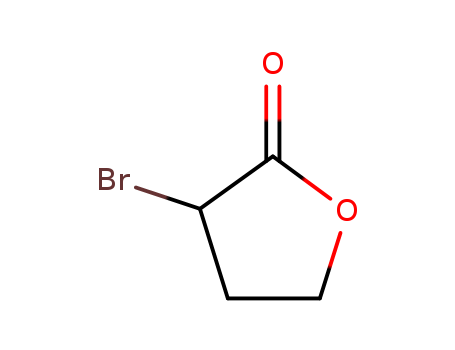
5061-21-2
- Product Name:2-Bromo-4-butanolide
- Molecular Formula:C4H5BrO2
- Purity:98% GC
- Molecular Weight:164.986
Product Details
Factory Supply industrial standard 2-Bromo-4-butanolide 5061-21-2 In Stock
- Molecular Formula:C4H5BrO2
- Molecular Weight:164.986
- Appearance/Colour:pale yellow liquid
- Vapor Pressure:0.00242mmHg at 25°C
- Refractive Index:n20/D 1.508(lit.)
- Boiling Point:287.9 °C at 760 mmHg
- Flash Point:111.5 °C
- PSA:26.30000
- Density:1.82 g/cm3
- LogP:0.69680
2-Bromo-4-butanolide(Cas 5061-21-2) Usage
InChI:InChI=1/C4H5BrO2/c5-3-1-2-7-4(3)6/h3H,1-2H2/t3-/m0/s1
5061-21-2 Relevant articles
Preparation method of DL-hydroxy selenomethionine
-
Paragraph 0008; 0016-0015, (2021/06/02)
The invention belongs to the field of pr...
A two-phase bromination process using tetraalkylammonium hydroxide for the practical synthesis of α-bromolactones from lactones
Hosono, Kazumi,Kodama, Shintaro,Nomoto, Akihiro,Ochi, Takanori,Ogawa, Akiya,Tabuchi, Akihiro,Yamamoto, Yuki,Yamazaki, Kento
supporting information, p. 2906 - 2914 (2022/01/12)
A simple and efficient method for α-brom...
Synthetic method for glufosinate ammonium
-
Paragraph 0034; 0035, (2019/02/04)
The invention relates to a synthetic met...
A method of manufacturing a brominated lacton compd.
-
Paragraph 0052; 0079-0081; 0082; 0085; 0086; 0088, (2018/11/22)
PROBLEM TO BE SOLVED: To provide a metho...
5061-21-2 Process route
-
-
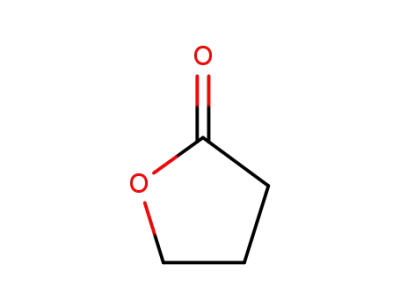
96-48-0
4-butanolide

-
-
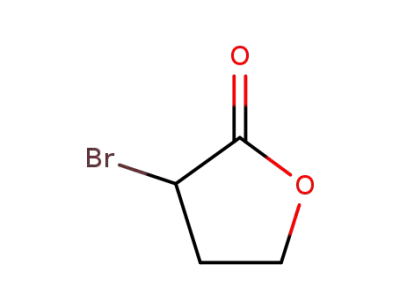
5061-21-2
α-bromo-γ-butyrolactone
| Conditions | Yield |
|---|---|
|
With
sulfur; bromine;
at 40 - 110 ℃;
for 4h;
Temperature;
Reagent/catalyst;
Inert atmosphere;
|
93.1% |
|
With
phosphorus; bromine;
at 80 ℃;
for 3h;
Cooling with ice;
|
88.7% |
|
With
bromine;
In
water;
|
75% |
|
With
phosphorus; bromine;
|
69% |
|
With
bromine; phosphorus tribromide;
at 100 ℃;
for 25h;
|
65% |
|
With
phosphorus; bromine;
at 80 ℃;
for 4h;
Cooling with ice;
|
43% |
|
4-butanolide;
With
bromine;
In
water;
Further stages.;
Heating;
|
|
|
4-butanolide;
With
phosphorus tribromide;
at 0 ℃;
With
bromine;
at 99 ℃;
|
|
|
With
bromine; phosphorus tribromide;
at 100 ℃;
for 4h;
Inert atmosphere;
|
-
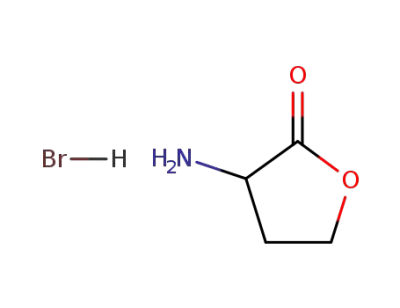
-
6305-38-0
α-amino-γ-butyrolactone hydrobromide

-
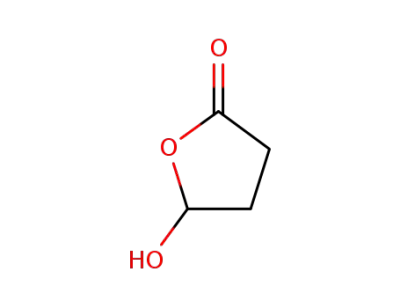
-
50768-69-9
5-hydroxydihydrofuran-2(3H)-one

-

-
5061-21-2
α-bromo-γ-butyrolactone

-
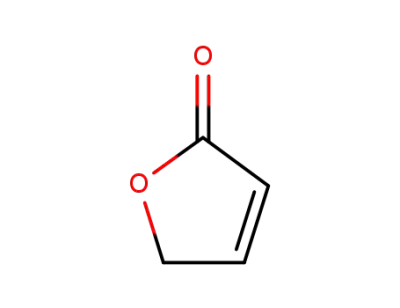
-
497-23-4
2-buten-4-olide
| Conditions | Yield |
|---|---|
|
With
5 % platinum on carbon; nitrogen(II) oxide;
In
water;
at 60 ℃;
for 25h;
under 15201 Torr;
Time;
Inert atmosphere;
|
5061-21-2 Upstream products
-
52412-07-4
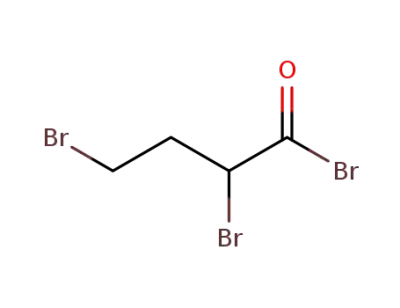
2,4-dibromo-butyryl bromide
-
96-48-0

4-butanolide
-
6305-38-0

α-amino-γ-butyrolactone hydrobromide
-
63164-16-9
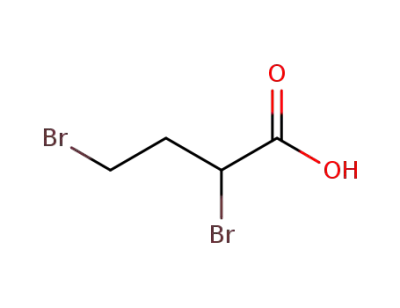
2,4-dibromo-butyric acid
5061-21-2 Downstream products
-
42473-02-9
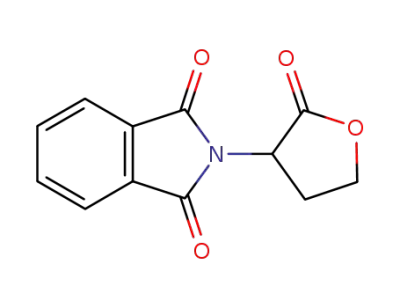
2-(2-oxotetrahydrofuran-3-yl)isoindolin-1,3-dione
-
31332-88-4
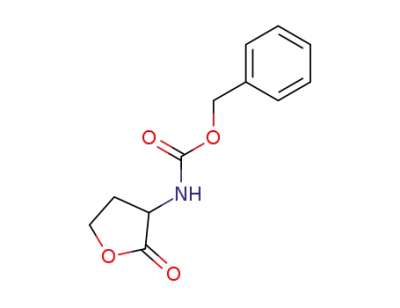
(S)-3-(N-benzyloxycarbonylamino)tetrahydrofuran-2-one
-
1192-20-7
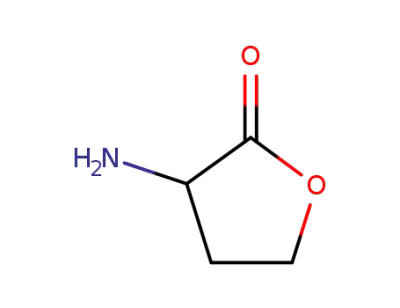
2-aminobutyrolactone
-
13602-48-7
3-benzoylamino-dihydro-furan-2-one
Relevant Products
-
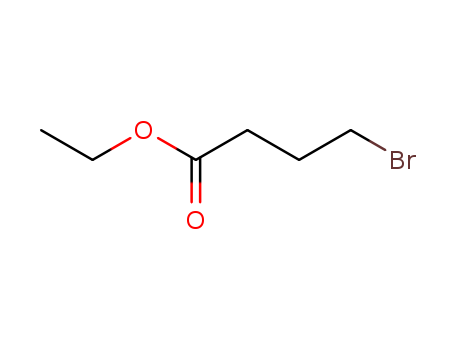
Ethyl 4-bromobutyrate
CAS:2969-81-5
-
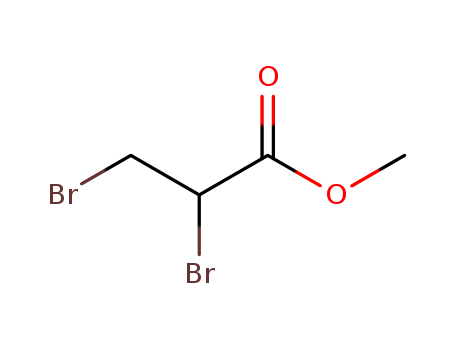
Methyl 2,3-dibromopropionate
CAS:1729-67-5
-
4-Nitrophenethyl bromide
CAS:5339-26-4