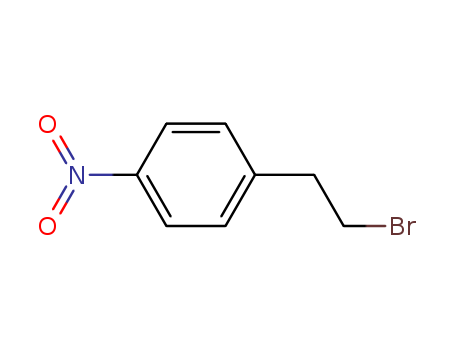
5339-26-4
- Product Name:4-Nitrophenethyl bromide
- Molecular Formula:C8H8BrNO2
- Purity:98% GC
- Molecular Weight:230.061
Product Details
Chinese factory supply 4-Nitrophenethyl bromide 5339-26-4 in stock with high standard
- Molecular Formula:C8H8BrNO2
- Molecular Weight:230.061
- Appearance/Colour:Slightly yellow solid.
- Vapor Pressure:0.00115mmHg at 25°C
- Melting Point:67-69 °C(lit.)
- Refractive Index:1.595
- Boiling Point:309.6 °C at 760 mmHg
- Flash Point:141 °C
- PSA:45.82000
- Density:1.562g/cm3
- LogP:3.05540
4-Nitrophenethyl bromide(Cas 5339-26-4) Usage
|
Biochem/physiol Actions |
4-Nitrophenethyl bromide is used as a marker in for the θ-class of glutathione S-transferases (GSTs). It activates the human θ-class glutathione transferase T1-1 enzyme. |
|
General Description |
4-Nitrophenethyl bromide (also known as p-nitrophenethyl bromide) is a nitroaromatic compound that undergoes intramolecular electron transfer upon one-electron reduction, forming an anion radical. This intermediate facilitates dehalogenation, releasing bromide ions, with the reaction rate influenced by factors such as C-Br bond dissociation energy and structural features. The study highlights that substitution patterns and the spatial relationship between the nitro group and the bromoethyl moiety play a role in the efficiency of electron transfer and subsequent dehalogenation. |
InChI:InChI=1/C8H8BrNO2/c9-6-5-7-1-3-8(4-2-7)10(11)12/h1-4H,5-6H2
5339-26-4 Relevant articles
Scalable anti-Markovnikov hydrobromination of aliphatic and aromatic olefins
Galli, Marzia,Fletcher, Catherine J.,Del Pozo, Marc,Goldup, Stephen M.
supporting information, p. 5622 - 5626 (2016/07/06)
To improve access to a key synthetic int...
Bimodal ligands with macrocyclic and acyclic binding moieties, complexes and compositions thereof, and methods of using
-
Page/Page column 109; 110; 137 - 139, (2015/09/23)
Substituted 1,4,7-triazacyclononane-N,N′...
Synthesis and biological evaluation of a new series of ebselen derivatives as glutathione peroxidase (GPx) mimics and cholinesterase inhibitors against Alzheimer's disease
Luo, Zonghua,Liang, Liang,Sheng, Jianfei,Pang, Yanqing,Li, Jianheng,Huang, Ling,Li, Xingshu
supporting information, p. 1355 - 1361 (2014/03/21)
A series of ebselen derivatives were des...
ANION RECEPTOR, AND ELECTROLYTE USING THE SAME
-
Page/Page column 31-32, (2008/06/13)
Disclosed is a novel anion receptor and ...
5339-26-4 Process route
-
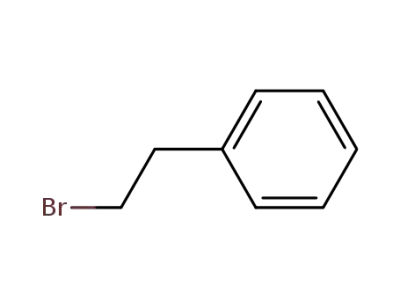
-
103-63-9
1-phenyl-2-bromoethane

-
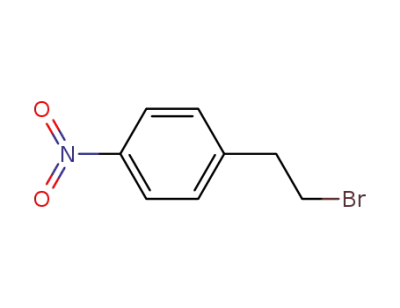
-
5339-26-4
(4-nitrophenyl)ethyl bromide
| Conditions | Yield |
|---|---|
|
|
30% |
|
With
nitric acid;
|
30% |
|
With
nitric acid;
at -70 ℃;
|
|
|
Nitrierung;
|
|
|
With
nitric acid; acetic anhydride; Nitrogen dioxide;
In
acetic acid;
|
|
|
(nitration);
|
|
|
With
nitric acid; acetic acid;
at -5 - 0 ℃;
for 5.5h;
|
|
|
With
sulfuric acid; nitric acid;
In
dichloromethane;
at 0 - 20 ℃;
for 4h;
|
|
|
With
nitric acid; acetic anhydride; acetic acid;
for 4h;
|
-
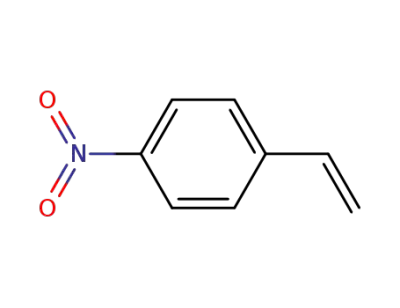
-
100-13-0
4-nitrostyrene

-

-
5339-26-4
(4-nitrophenyl)ethyl bromide
| Conditions | Yield |
|---|---|
|
With
2,2'-azobis(isobutyronitrile); hydrogen bromide;
In
toluene;
at 0 ℃;
for 2h;
|
73% |
5339-26-4 Upstream products
-
103-63-9
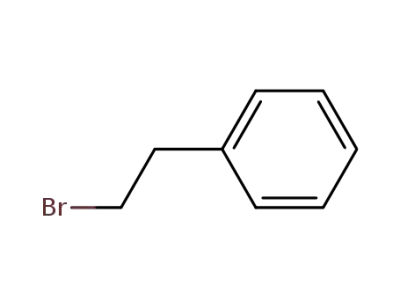
1-phenyl-2-bromoethane
-
100-27-6
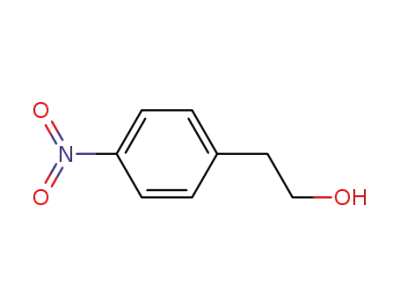
2-(4-nitrophenyl)ethanol
-
104-03-0
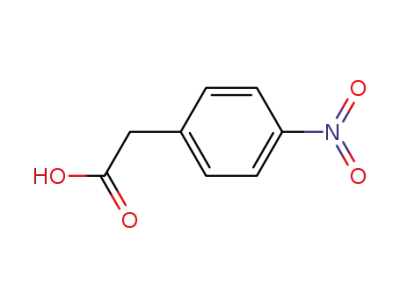
4-nitrobenzeneacetic acid
-
50434-36-1
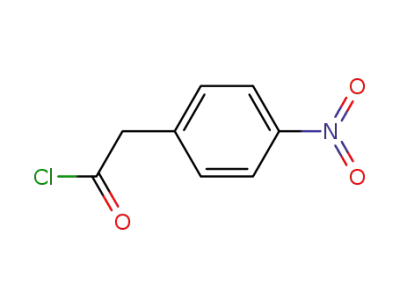
4-nitrophenylacetyl chloride
5339-26-4 Downstream products
-
101496-66-6
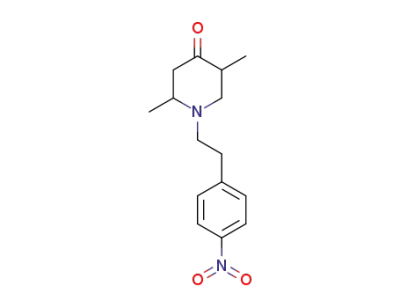
2,5-dimethyl-1-(4-nitro-phenethyl)-piperidin-4-one
-
101291-45-6
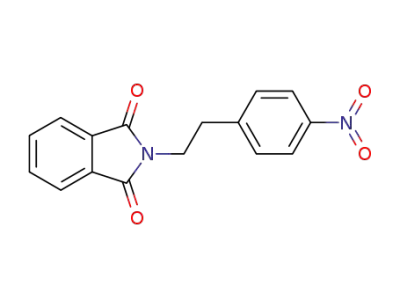
N-<2-(4-nitrophenyl)ethyl>phthalimide
-
119438-76-5
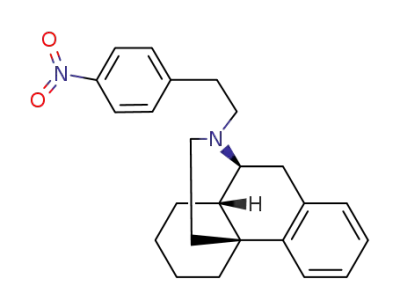
rac-17-(4-nitro-phenethyl)-morphinane
-
1027357-11-4
methyl-(4-nitro-phenethyl)-sulfone
Relevant Products
-
2-Bromoacetamide
CAS:683-57-8
-
2-Bromo-4-butanolide
CAS:5061-21-2
-
Methyl 2-methoxybenzoate
CAS:606-45-1